In an ideal world, fuel will burn efficiently to produce just CO2 (carbon dioxide), H20 (water) and N2 (nitrogen), but in reality this never happens. Those three compounds are not toxic to humans and therefore the ever increasing strictness of emissions standards aims to encourage vehicle manufacturers to improve their engines to get as close as possible to this goal. New cars must conform to a standard called Euro 6 which came into force in September 2015. Compared to the original 1992 limits (Euro 1) some pollutant levels are reduced by up to 96%!
Euro 1 emission limits (1992):
- CO – 2.72 g/km (Petrol and diesel)
- HC+ NOx – 0.97 g/km (Petrol and diesel)
- PM (volume) – 0.14 g/km (diesel only)
Euro 6 emission limits (2015) (petrol):
- CO – 1.0 g/km
- HC – 0.10 g/km
- NOx – 0.06 g/km
- NMHC – 0.0068 g/km
- PM (volume) – 0.005 g/km (Direct Injection only)
- PM (number) – 6.0×10 ^11/km (Direct Injection only)
Euro 6 emission limits (diesel):
- CO – 0.50 g/km
- HC+ NOx – 0.17 g/km
NOx – 0.08 g/km - PM – 0.005 g/km
- PM – 6.0×10 ^11/km
CO = carbon monoxide; HC = hydrocarbons; NOx = nitrogen oxides; PM = particulate matter and number of particles; NMHC = non-methane hydrocarbons. See explanation below.
Types of gases and particulates that come from vehicle exhausts
Carbon monoxide is colourless, odourless and tasteless. It’s toxic to animals above concentrations of about 35 parts per million, but it does occur naturally at low levels. It forms a temporary atmospheric pollutant until it combines to form ground-level ozone. It is produced when fuel isn’t burned fully.
Nitrogen oxides (NOx) which refers to NO and NO2 are released by vehicle exhausts. They’re produced during high-temperature combustion and are harmful to human health. It can cause acid rain. They react with hydrocarbons to produce ground-level ozone and contribute to the formation of particulate matter in the atmosphere. The gas is an irritant to lungs and can trigger asthma attacks.
Hydrocarbons are one of the causes of smog. They are produced when fuel is not fully burned and through evaporation of fuel when it’s in the tank.
Particulates are tiny particles like diesel soot that are released into the air. They are damaging to lungs and contribute to smog.
Benzene (C6H6) is emitted in unburnt fuel as it’s naturally occurring in small quantities. It’s toxic and carcinogenic and long-term exposure is linked with leukemia.
Issues with older types of fuel
Sulphur dioxide contributes to the formation of ozone and particulate matter. It can affect the performance of catalytic converters. It is removed from petrol and diesel during the refining process.
Lead used to be added to fuel to form leaded petrol. It’s the reason why our fuel today is called unleaded petrol and not just petrol. Lead interferes with the normal production of red blood cells. It’s no longer added to petrol and therefore doesn’t appear in exhaust emissions.
Tests to determine emissions
When you take your vehicle for an MOT its exhaust emissions will be checked. The testing procedure can be found here.
Technology to reduce exhaust emissions
Emissions standards were first implemented in 1970 with the first standard, but didn’t really make much headway until 1992 with Euro 1 which made catalytic converters mandatory on petrol cards to reduce carbon monoxide (CO) emissions. This also meant that leaded fuel would no longer be able to be sold as the lead affected the catalytic converters. Euro 1 had similar emissions levels for petrol and diesel but Euro 2 required different levels of reductions.
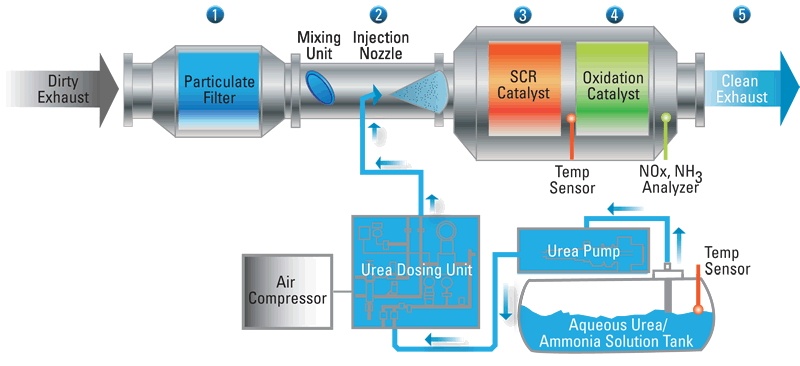
Technological improvements introduced by engine manufacturers includes carbon canisters to hold evaporated fuel vapours. NOx is converted to N2 using Selective Catalytic Reduction (SCR). Hydrocarbons and carbon monoxide are reduced using oxicats (a substrate made of thousands of small channels coated with highly porous catalysts like palladium and platinum); the result is carbon dioxide and water. Diesel particulate filters are used to filter out soot and other particles. This diagram shows how the system works: